Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
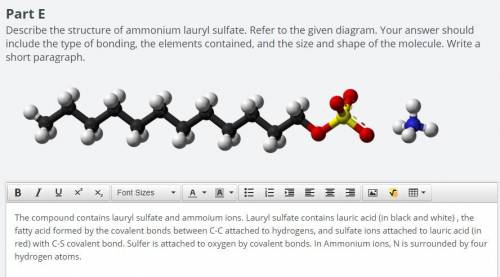
Explain how the structure of ammonium lauryl sulfate, as described in parts E and F, produces the pr...
Questions

World Languages, 14.10.2020 19:01


Biology, 14.10.2020 19:01



Mathematics, 14.10.2020 19:01

Geography, 14.10.2020 19:01

Mathematics, 14.10.2020 19:01



Mathematics, 14.10.2020 19:01

Mathematics, 14.10.2020 19:01

Biology, 14.10.2020 19:01

History, 14.10.2020 19:01



Mathematics, 14.10.2020 19:01


Mathematics, 14.10.2020 19:01