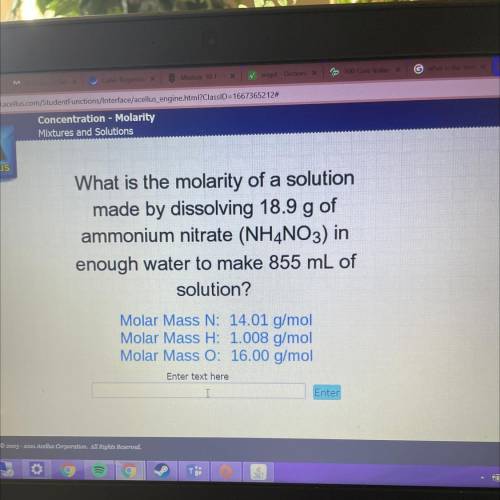
What is the molarity of a solution
made by dissolving 18.9 g of
ammonium nitrate (NH4NO3) in<...

Chemistry, 27.01.2021 20:40 andrewmena05
What is the molarity of a solution
made by dissolving 18.9 g of
ammonium nitrate (NH4NO3) in
enough water to make 855 mL of
solution?
Molar Mass N: 14.01 g/mol
Molar Mass H: 1.008 g/mol
Molar Mass O: 16.00 g/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

You know the right answer?
Questions

History, 26.08.2019 20:30


Mathematics, 26.08.2019 20:30





English, 26.08.2019 20:30

History, 26.08.2019 20:30

English, 26.08.2019 20:30



Biology, 26.08.2019 20:30

Mathematics, 26.08.2019 20:30




Mathematics, 26.08.2019 20:30

Spanish, 26.08.2019 20:30



