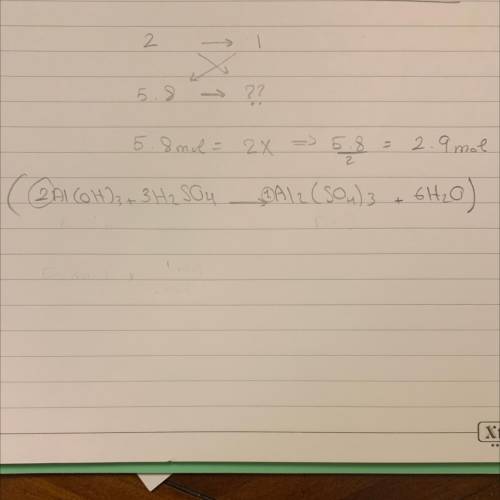Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Based on the equation below, how many moles of aluminum sulfate (Al2(SO4)3) will be produced from th...
Questions


Business, 23.01.2021 05:50

Business, 23.01.2021 05:50







History, 23.01.2021 05:50


Mathematics, 23.01.2021 05:50

Physics, 23.01.2021 05:50

Mathematics, 23.01.2021 05:50




Mathematics, 23.01.2021 05:50

Mathematics, 23.01.2021 05:50

Mathematics, 23.01.2021 05:50