
Chemistry, 27.01.2021 09:10 thornlilly17
Which two chemicals are products in the following chemical reaction?
PbO2 + 4HCI — PbCl2 + Cl2 + 2H20
A. PbCl2
B. HCI
C. Cl2
D. PbO2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3
You know the right answer?
Which two chemicals are products in the following chemical reaction?
PbO2 + 4HCI — PbCl2 + Cl2 + 2H...
Questions



Chemistry, 27.10.2020 03:40


Mathematics, 27.10.2020 03:40

Mathematics, 27.10.2020 03:40

Mathematics, 27.10.2020 03:40




Chemistry, 27.10.2020 03:40

English, 27.10.2020 03:40



Mathematics, 27.10.2020 03:40

Physics, 27.10.2020 03:40




Geography, 27.10.2020 03:40

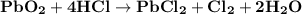
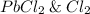 are the products in the given chemical reaction.
are the products in the given chemical reaction. 

