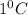Which best explains why water has a high specific heat? hydrogen bonds decrease the amount of energy that is required for the temperature to change. hydrogen bonds increase the amount of energy that is required for the temperature to change. ion-dipole interactions increase the amount of energy that is required for the temperature to change. ion-dipole interactions decrease the amount of energy that is required for the temperature to change.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
Which best explains why water has a high specific heat? hydrogen bonds decrease the amount of energ...
Questions


Mathematics, 04.12.2020 18:00

Biology, 04.12.2020 18:00

Mathematics, 04.12.2020 18:00

English, 04.12.2020 18:00


Mathematics, 04.12.2020 18:00

Mathematics, 04.12.2020 18:00



Biology, 04.12.2020 18:00


Physics, 04.12.2020 18:00

Mathematics, 04.12.2020 18:00



Biology, 04.12.2020 18:00

English, 04.12.2020 18:00


History, 04.12.2020 18:00