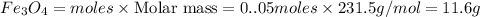Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8....

Chemistry, 26.01.2021 01:10 lazavionadams81
Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8.4 g of Fe, how many of Fe 3O4 are formed?
a) Calculate the limiting reactant
b) Calculate the number of grams of Al produced.
c) Calculate the number of grams of Fe3O4 produced.
d) Calculate the percent yield if 10g of Fe O4 were obtained?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
Questions

Physics, 18.07.2019 16:30

English, 18.07.2019 16:30


Chemistry, 18.07.2019 16:30



Chemistry, 18.07.2019 16:30

Biology, 18.07.2019 16:30




History, 18.07.2019 16:30

Mathematics, 18.07.2019 16:30

Business, 18.07.2019 16:30


Chemistry, 18.07.2019 16:30

English, 18.07.2019 16:30

Biology, 18.07.2019 16:30


Social Studies, 18.07.2019 16:30

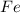 is the limiting reagent
is the limiting reagent 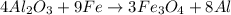
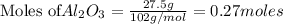
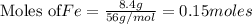
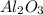
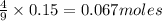 of
of 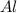
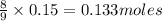 of
of 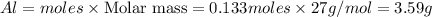
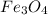
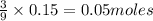 of
of