
Chemistry, 01.10.2019 05:00 coryintheswamp
Urgent: how is a pure substance different from a mixture?
a. pure substances are separated by physical means
b. mixtures are made up of more than one component
c. a pure substance is heterogeneous
d. mixtures cannot be separated by physical means.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Urgent: how is a pure substance different from a mixture?
a. pure substances are separated b...
a. pure substances are separated b...
Questions

Geography, 25.01.2021 23:50


Social Studies, 25.01.2021 23:50

Mathematics, 25.01.2021 23:50



Mathematics, 25.01.2021 23:50





Computers and Technology, 25.01.2021 23:50



Chemistry, 25.01.2021 23:50


Mathematics, 25.01.2021 23:50


Biology, 25.01.2021 23:50

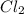 and
and 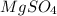 are pure substances.
are pure substances.

