
Chemistry, 25.01.2021 21:40 911wgarcia
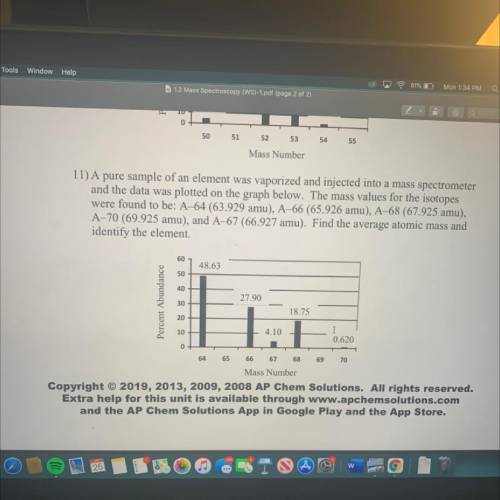
11) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data was plotted on the graph below. The mass values for the isotopes
were found to be: A–64 (63.929 amu), A–66 (65.926 amu), A-68 (67.925 amu),
A-70 (69.925 amu), and A-67 (66.927 amu). Find the average atomic mass and
identify the element.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
11) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data wa...
Questions

Mathematics, 02.03.2021 20:10


SAT, 02.03.2021 20:10

Mathematics, 02.03.2021 20:10

Engineering, 02.03.2021 20:10


English, 02.03.2021 20:10

Mathematics, 02.03.2021 20:10

Biology, 02.03.2021 20:10


Law, 02.03.2021 20:10





Mathematics, 02.03.2021 20:10


History, 02.03.2021 20:10


Mathematics, 02.03.2021 20:10



