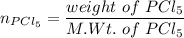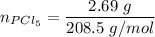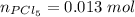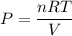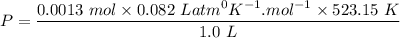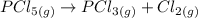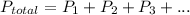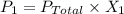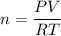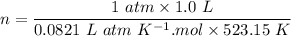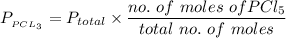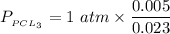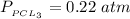Chemistry, 25.01.2021 21:10 Queenbee2304
A sample of PCl5 weighting 2.69 gram was placed in 1.00 Litter container and completely vaporized at 250C. The pressure observed at that temperature was 1.00 atm. The possibility exists that some of the PCl5 dissociated according to PCl5 (g) ! PCl3 (g) Cl2 (g) . What must be the partial pressures of PCl5 PCl3 and Cl2 under these experimental conditions

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 07:30
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
A sample of PCl5 weighting 2.69 gram was placed in 1.00 Litter container and completely vaporized at...
Questions

Mathematics, 23.01.2021 05:20

Mathematics, 23.01.2021 05:20

Computers and Technology, 23.01.2021 05:20

Biology, 23.01.2021 05:20


Mathematics, 23.01.2021 05:20


Mathematics, 23.01.2021 05:20


Mathematics, 23.01.2021 05:20


Mathematics, 23.01.2021 05:20

Mathematics, 23.01.2021 05:20

Social Studies, 23.01.2021 05:20

Mathematics, 23.01.2021 05:20

Mathematics, 23.01.2021 05:20

Arts, 23.01.2021 05:20