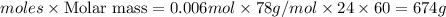The CO2 that builds up in the air of a submerged submarine can be removed by reacting it with sodium peroxide. 2 Na2O2 (s) + 2 CO2 (g) → 2 Na2CO3 (s) + O2 (g) If a sailor exhales 150.0 mL of CO2 per minute at 20oC and 1.02 atm, how much sodium peroxide is needed per sailor in a 24 hr period?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
The CO2 that builds up in the air of a submerged submarine can be removed by reacting it with sodium...
Questions

Mathematics, 03.02.2020 09:50

Mathematics, 03.02.2020 09:50

History, 03.02.2020 09:50

Chemistry, 03.02.2020 09:50

History, 03.02.2020 09:50



Chemistry, 03.02.2020 09:50

English, 03.02.2020 09:50

Biology, 03.02.2020 09:50

Social Studies, 03.02.2020 09:50

Physics, 03.02.2020 09:50



Biology, 03.02.2020 09:50

English, 03.02.2020 09:50

Mathematics, 03.02.2020 09:50

Physics, 03.02.2020 09:50

Mathematics, 03.02.2020 09:50

English, 03.02.2020 09:50

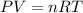
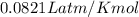
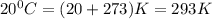
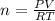
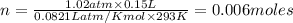
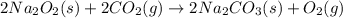
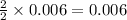 moles of sodium peroxide
moles of sodium peroxide