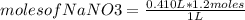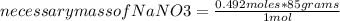Chemistry, 26.08.2019 14:30 haileysolis5
Alaboratory procedure calls for making 410.0ml of a 1.2m nano3 solution.
what mass of nano3 (in g) is needed?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Alaboratory procedure calls for making 410.0ml of a 1.2m nano3 solution.
what mass of nano3 (i...
what mass of nano3 (i...
Questions


SAT, 05.01.2022 07:40

SAT, 05.01.2022 07:40

Business, 05.01.2022 07:40

Chemistry, 05.01.2022 07:40

Physics, 05.01.2022 07:40


Arts, 05.01.2022 07:50


Social Studies, 05.01.2022 07:50


Physics, 05.01.2022 07:50



Mathematics, 05.01.2022 07:50



Mathematics, 05.01.2022 07:50