
Chemistry, 24.01.2021 22:20 mlguerrero12
Zn + 2HCI -> ZnCl2 + H2
a. How many moles of ZnCl, can be made from 1.7g of Zn?
b. If 6.4 grams of HCl are used in this reaction, how many moles of ZnCl, are
produced?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a planet rotates 360 degrees during a 24 hour time period, what does that tell us about the planet? a. the middle of the planet is in darkness b. the seasons on the planet vary every day. c. the planet runs on a 12-hour time clock. d. the temperature on the planet varies daily.
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
Zn + 2HCI -> ZnCl2 + H2
a. How many moles of ZnCl, can be made from 1.7g of Zn?
b. I...
b. I...
Questions

Mathematics, 02.10.2019 07:00


Mathematics, 02.10.2019 07:00



Geography, 02.10.2019 07:00



English, 02.10.2019 07:00

History, 02.10.2019 07:00

History, 02.10.2019 07:00


History, 02.10.2019 07:00

History, 02.10.2019 07:00


Mathematics, 02.10.2019 07:00

Mathematics, 02.10.2019 07:00


Health, 02.10.2019 07:00

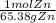 = 0.0273784032 moles Zn
= 0.0273784032 moles Zn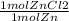 = 0.0273784032 moles ZnCl2
= 0.0273784032 moles ZnCl2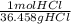 = 0.1755444621 mol HCl
= 0.1755444621 mol HCl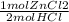 = 0.877722311 moles ZnCl2 are produced.
= 0.877722311 moles ZnCl2 are produced.

