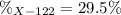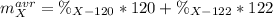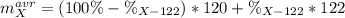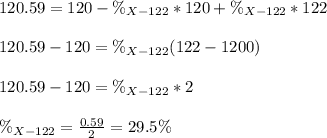Chemistry, 23.01.2021 07:40 Rockey3876
An unknown element is a mixture of isotopes ¹²⁰X and ¹²²X. The average atomic mass of X is 120.59 amu. What is the percent abundance of ¹²²X?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1

Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
An unknown element is a mixture of isotopes ¹²⁰X and ¹²²X. The average atomic mass of X is 120.59 am...
Questions


Geography, 31.08.2020 18:01



Health, 31.08.2020 18:01

English, 31.08.2020 18:01


English, 31.08.2020 18:01

Chemistry, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01

Geography, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01



Biology, 31.08.2020 18:01


Business, 31.08.2020 18:01