
Chemistry, 23.01.2021 06:50 johnnyhalusewa
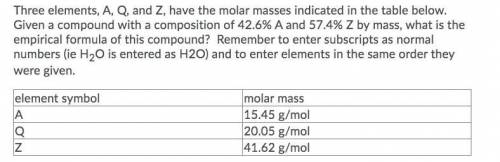
Three elements, A, Q, and Z, have the molar masses indicated in the table below. Given a compound with a composition of 42.6% A and 57.4% Z by mass, what is the empirical formula of this compound? Remember to enter subscripts as normal numbers (ie H2O is entered as H2O) and to enter elements in the same order they were given.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Three elements, A, Q, and Z, have the molar masses indicated in the table below. Given a compound wi...
Questions

Biology, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10


Physics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10



Social Studies, 18.03.2021 01:10



English, 18.03.2021 01:10





