
Chemistry, 22.01.2021 20:20 orlando979
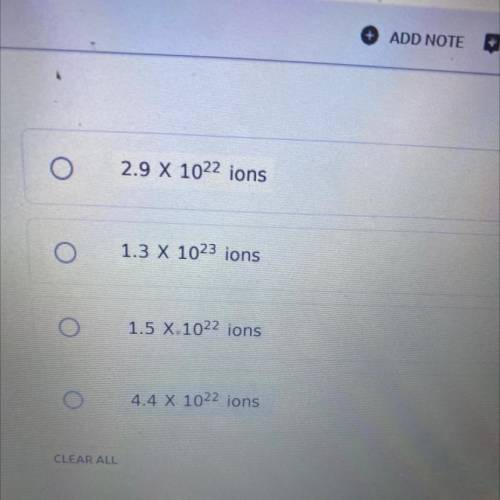
How many total ions are there in 3.6 grams of Mg(NO3)2 ? The molar mass of magnesium nitrate is 148.3 g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
How many total ions are there in 3.6 grams of Mg(NO3)2 ? The molar mass of
magnesium nitrate is 148...
Questions

Business, 12.01.2021 16:20

Mathematics, 12.01.2021 16:20

Social Studies, 12.01.2021 16:20


Mathematics, 12.01.2021 16:20



Mathematics, 12.01.2021 16:20

Physics, 12.01.2021 16:20

Business, 12.01.2021 16:20

Business, 12.01.2021 16:20


Mathematics, 12.01.2021 16:20

Mathematics, 12.01.2021 16:20


Mathematics, 12.01.2021 16:20

Mathematics, 12.01.2021 16:20


Advanced Placement (AP), 12.01.2021 16:20

Mathematics, 12.01.2021 16:20



