Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1


Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
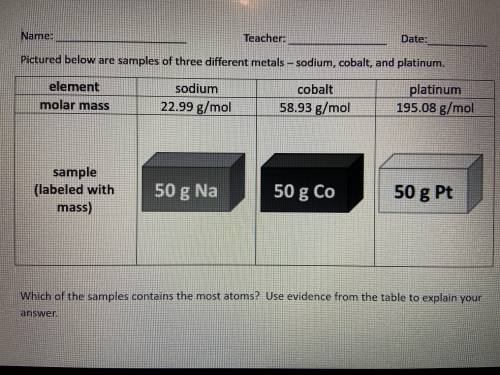
Which of the samples contains the most atoms? Use evidence from the table to explain your answer.
Questions

English, 05.10.2020 06:01

Biology, 05.10.2020 06:01


Chemistry, 05.10.2020 07:01



Arts, 05.10.2020 07:01


Mathematics, 05.10.2020 07:01


Mathematics, 05.10.2020 07:01

Chemistry, 05.10.2020 07:01

Mathematics, 05.10.2020 07:01

Mathematics, 05.10.2020 07:01


English, 05.10.2020 07:01

English, 05.10.2020 07:01


English, 05.10.2020 07:01

Mathematics, 05.10.2020 07:01