
1. Write the balanced chemical equation for the reaction you are performing.
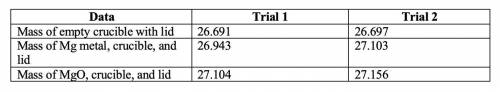
2. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and lid (row 2 in the chart) to find the mass of magnesium for each trial.
• Trial 1: 0.252
• Trial 2: 0.406
3. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual yield of magnesium oxide for each trial.
• Trial 1: 0.413
• Trial 2: 0.459
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
• Trial 1:
• Trial 2:
5. Determine the percent yield of MgO for your experiment for each trial.
• Trial 1:
• Trial 2:
6. Determine the average percent yield of MgO for the two trials.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
1. Write the balanced chemical equation for the reaction you are performing.
2. Subtract the mass o...
Questions

Mathematics, 15.01.2020 20:31

Mathematics, 15.01.2020 20:31



Physics, 15.01.2020 20:31





Chemistry, 15.01.2020 20:31





Business, 15.01.2020 20:31


Mathematics, 15.01.2020 20:31

Mathematics, 15.01.2020 20:31





