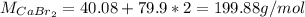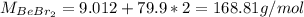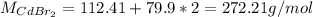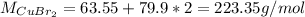Beryllium (Be) 9.012

Chemistry, 21.01.2021 22:40 ehuntsman8221
Calculate the molar mass of each salt.
Element Molar mass (g/mol)
Beryllium (Be) 9.012
Magnesium (Mg) 24.31
Cobalt (Co) 58.93
Cadmium (Cd) 112.41
Iodine (II) 126.90
a. The Molar mass of CaBr2 is:
b. The Molar mass of BeBr2 is:
c. The Molar mass of CdBr2 is:
d. The Molar mass of CuBr2 is:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
Calculate the molar mass of each salt.
Element Molar mass (g/mol)
Beryllium (Be) 9.012
Beryllium (Be) 9.012
Questions







French, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01


History, 16.10.2020 03:01

History, 16.10.2020 03:01

History, 16.10.2020 03:01

Biology, 16.10.2020 03:01



English, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01


Biology, 16.10.2020 04:01