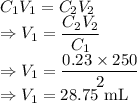Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
A stock solution of Al(CH3COO)3 is available to prepare solutions that are more dilute. Calculate th...
Questions




English, 26.10.2020 20:20

History, 26.10.2020 20:20


Mathematics, 26.10.2020 20:20


Mathematics, 26.10.2020 20:20

Mathematics, 26.10.2020 20:20




Mathematics, 26.10.2020 20:20

Geography, 26.10.2020 20:20


History, 26.10.2020 20:20

History, 26.10.2020 20:20

Mathematics, 26.10.2020 20:20

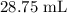
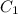 = Initial concentration = 2 M
= Initial concentration = 2 M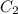 = Final concentration = 0.23 M
= Final concentration = 0.23 M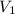 = Initial volume
= Initial volume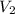 = Final volume = 250 mL
= Final volume = 250 mL