Chemistry, 21.01.2021 22:10 Gabysh4105
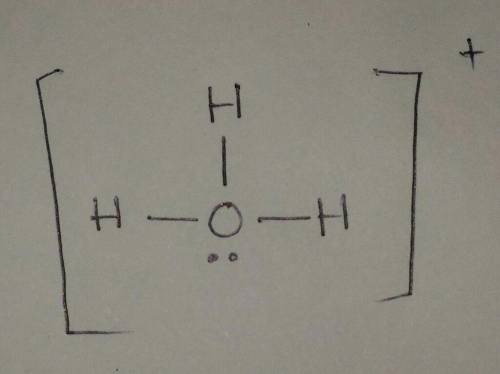
Draw a Lewis structure for H3O+ . Include all hydrogen atoms and show all unshared electrons and the formal charges, if any. Assume that bonding follows the octet rule.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Draw a Lewis structure for H3O+ . Include all hydrogen atoms and show all unshared electrons and the...
Questions

Chemistry, 28.10.2020 21:30






Mathematics, 28.10.2020 21:30





Mathematics, 28.10.2020 21:30

Chemistry, 28.10.2020 21:30

Geography, 28.10.2020 21:30

English, 28.10.2020 21:30

History, 28.10.2020 21:30



Mathematics, 28.10.2020 21:30

Mathematics, 28.10.2020 21:30