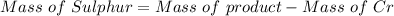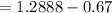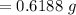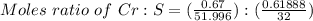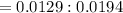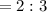Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1


Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3
You know the right answer?
A 0.67 gram sample of chromium is reacted with sulfur. The resulting chromium sulfide has a mass of...
Questions


Biology, 05.04.2021 23:10






English, 05.04.2021 23:10

Mathematics, 05.04.2021 23:10




Chemistry, 05.04.2021 23:10

Social Studies, 05.04.2021 23:10



History, 05.04.2021 23:10

History, 05.04.2021 23:10


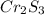 ".
".