
Chemistry, 21.01.2021 21:40 serenityarts123
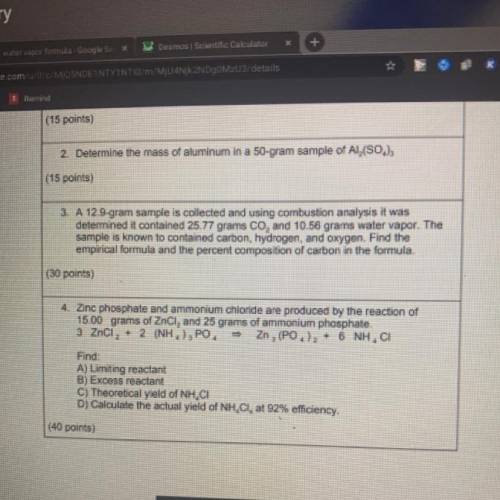
4. Zinc phosphate and ammonium chlonde are produced by the reaction of
15.00 grams of Encl, and 25 grams of ammonium phosphate
3 Zng + NH, E. Zn, (PO.), + 6 NH.
Find:
A) Limiting reactant
B) Excess reactant
c) Theoretical yeld of NH, CI
D) Calculate the actual yield of NH Cl, at 92% efficiency.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
4. Zinc phosphate and ammonium chlonde are produced by the reaction of
15.00 grams of Encl, and 25...
Questions


Mathematics, 06.05.2021 22:30

Mathematics, 06.05.2021 22:30



English, 06.05.2021 22:30


Mathematics, 06.05.2021 22:30



Chemistry, 06.05.2021 22:30


Mathematics, 06.05.2021 22:30

Chemistry, 06.05.2021 22:30


Biology, 06.05.2021 22:30

Mathematics, 06.05.2021 22:30


English, 06.05.2021 22:30




