
Chemistry, 21.01.2021 08:50 raymond5799
8. For each of the following pairs of atoms, highlight the atom that will attract shared electrons more
strongly.
a. Carbon or
Chlorine
b. Rubidium or Bromine
C. lodine or Indium
d. Silver
Sulfur
e. Arsenic
or
Sodium
f. Hydrogen or Selenium
or
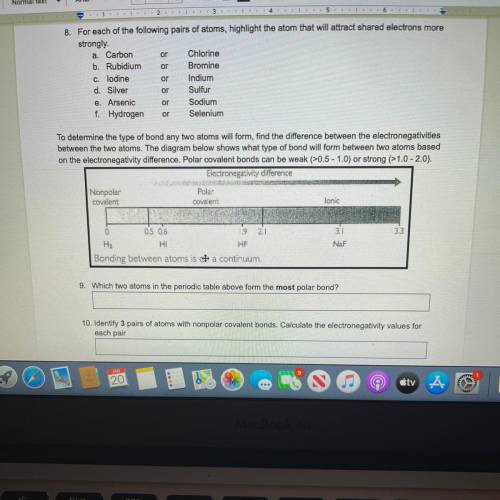
To determine the type of bond any two atoms will form, find the difference between the electronegativities
between the two atoms. The diagram below shows what type of bond will form between two atoms based
on the electronegativity difference. Polar covalent bonds can be weak (>0.5 - 1.0) or strong (>1.0-2.0).
Electronegativity difference
Nonpolar
Polas
covalent
covalent
lonic
0
2
3.3
0.5 0.6
9
H2
HI
HF
Bonding between atoms is a continuum.
3.1
NaF
9. Which two atoms in the periodic table above form the most polar bond?
10. Identify 3 pairs of atoms with nonpolar covalent bonds. Calculate the electronegativity values
each pair

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
8. For each of the following pairs of atoms, highlight the atom that will attract shared electrons m...
Questions

English, 17.04.2020 01:33








Chemistry, 17.04.2020 01:34





Social Studies, 17.04.2020 01:34



Mathematics, 17.04.2020 01:34

Mathematics, 17.04.2020 01:34




