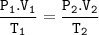Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
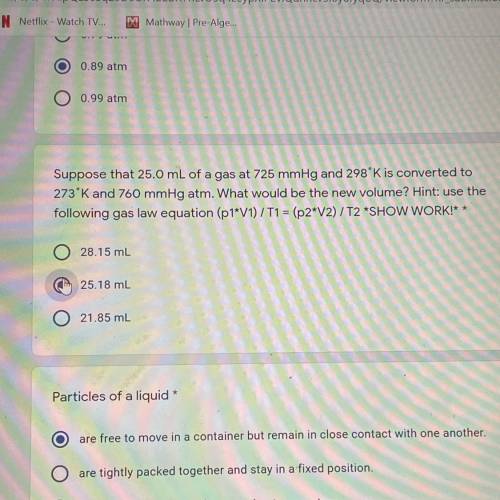
Suppose that 25,0 mL of a gas at 725 mmHg and 298K is converted to
273'K and 760 mmHg atm. What wou...
Questions









Mathematics, 11.02.2020 18:44



English, 11.02.2020 18:44

Social Studies, 11.02.2020 18:44


History, 11.02.2020 18:45

Engineering, 11.02.2020 18:45


Mathematics, 11.02.2020 18:45