

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
If 45.00 g of precipitate is formed from the reaction of 0.100 mol/L
HCL(aq) and 0.124 mol/L AgNO3(...
Questions

History, 12.04.2021 17:50

Mathematics, 12.04.2021 17:50





Mathematics, 12.04.2021 17:50



English, 12.04.2021 17:50


Spanish, 12.04.2021 17:50

Mathematics, 12.04.2021 17:50


Mathematics, 12.04.2021 17:50

English, 12.04.2021 17:50




Social Studies, 12.04.2021 17:50

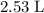 (rounded to three significant figures) assuming that
(rounded to three significant figures) assuming that 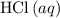 is in excess.
is in excess. 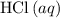 and
and 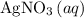 precipitate,
precipitate, 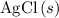 (the said precipitate) and
(the said precipitate) and 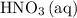 are produced:
are produced: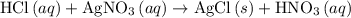 (verify that this equation is indeed balanced.)
(verify that this equation is indeed balanced.)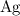 and
and 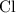 on a modern periodic table:
on a modern periodic table: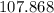 .
.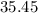 .
.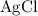 :
: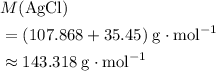 .
.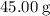 of this compound:
of this compound: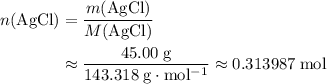 .
.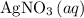 and
and 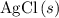 are both one.
are both one. 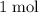 of
of 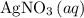 formula units to produce
formula units to produce 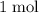 of
of 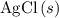 formula units.
formula units. 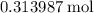 of
of 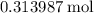 of
of 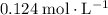 :
: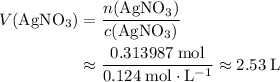 .
.

