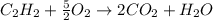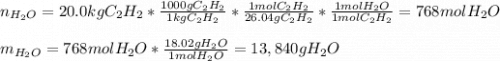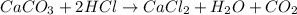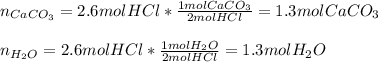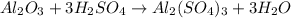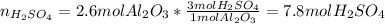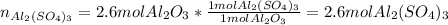Chemistry, 20.01.2021 14:00 matthewarroyo8988
Write the balanced equation and determine the information requested in each of the following.
1. The number of moles and the mass of water formed by the combustion of 20.0kg of acetylene, C2H2,in an excess of oxygen.
2. Limestone, caco3,dissolves in hydrochloric acid, Hcl, to form calcium chloride, carbon dioxide, and water. How many moles of Hcl are required to dissolve 2.6mol of caco3? How many moles of water are formed?
3.aluminiumoxide reacts with sulphuric acid to form aluminiumsulfate and water
A. how many moles of H2SO4 are required to react with 2.6mol of Al2O3
B. How many moles of Al2(So4)3 are formed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

You know the right answer?
Write the balanced equation and determine the information requested in each of the following.
1. Th...
Questions


Mathematics, 02.03.2021 21:00


Mathematics, 02.03.2021 21:00

Social Studies, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

History, 02.03.2021 21:00



Mathematics, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

English, 02.03.2021 21:00


Mathematics, 02.03.2021 21:00


Arts, 02.03.2021 21:00

English, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00