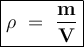Chemistry, 20.01.2021 08:00 runninglovexoxo
A student measures 48.8 mL of concentrated nitric acid, which has a density of 1.55g/ mL. What is the mass of the acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
A student measures 48.8 mL of concentrated nitric acid, which has a density of 1.55g/ mL. What is th...
Questions

Chemistry, 12.11.2019 06:31

History, 12.11.2019 06:31





Mathematics, 12.11.2019 06:31

Mathematics, 12.11.2019 06:31


Mathematics, 12.11.2019 06:31


History, 12.11.2019 06:31

Mathematics, 12.11.2019 06:31

Mathematics, 12.11.2019 06:31


History, 12.11.2019 06:31


Health, 12.11.2019 06:31