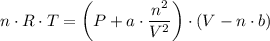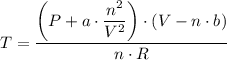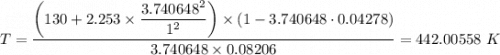Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

You know the right answer?
When 60.0 g methane (CH4) is placed in a 1.00-L vessel, the pressure is measured to be 130 atm. Calc...
Questions

Mathematics, 23.10.2020 20:20

Mathematics, 23.10.2020 20:20

History, 23.10.2020 20:20



Chemistry, 23.10.2020 20:20

Health, 23.10.2020 20:20

History, 23.10.2020 20:20

Mathematics, 23.10.2020 20:20


Mathematics, 23.10.2020 20:20

Mathematics, 23.10.2020 20:20

Mathematics, 23.10.2020 20:20

English, 23.10.2020 20:20

Social Studies, 23.10.2020 20:20



English, 23.10.2020 20:20

Biology, 23.10.2020 20:20

Health, 23.10.2020 20:20