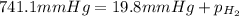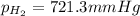Chemistry, 19.01.2021 21:10 alivas6618
(d) In the laboratory, the temperature is 295 K and the total pressure in the gas-collecting tube is 741.2 mmHg . If the vapor pressure of water at 295 K is 19.8 mmHg , determine the pressure of the H2(g) in the gas-collecting tube.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
(d) In the laboratory, the temperature is 295 K and the total pressure in the gas-collecting tube is...
Questions

Mathematics, 21.04.2021 08:40

Geography, 21.04.2021 08:40

Physics, 21.04.2021 08:40


Biology, 21.04.2021 08:40

History, 21.04.2021 08:40




Mathematics, 21.04.2021 08:40

Advanced Placement (AP), 21.04.2021 08:40









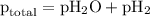
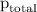 = total pressure of gases = 741.1 mm Hg
= total pressure of gases = 741.1 mm Hg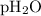 = partial pressure of water = 19.8 mm Hg
= partial pressure of water = 19.8 mm Hg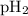 = partial pressure of hydrogen = ?
= partial pressure of hydrogen = ?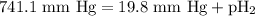
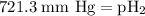
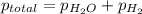
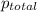 =total pressure of gases = 741.1 mm Hg
=total pressure of gases = 741.1 mm Hg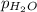 = partial pressure of water = 19.8 mm Hg
= partial pressure of water = 19.8 mm Hg 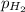 = partial pressure of hydrogen = ?
= partial pressure of hydrogen = ?