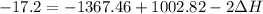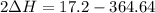Chemistry, 19.01.2021 19:40 supergraciepie
A scientist measures the standard enthalpy change for the following reaction to be -17.2 kJ : Ca(OH)2(aq) 2 HCl(aq)CaCl2(s) 2 H2O(l) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of HCl(aq) is kJ/mol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
A scientist measures the standard enthalpy change for the following reaction to be -17.2 kJ : Ca(OH)...
Questions



English, 16.11.2020 06:40

English, 16.11.2020 06:40


Health, 16.11.2020 06:40


Social Studies, 16.11.2020 06:40

Social Studies, 16.11.2020 06:40

Biology, 16.11.2020 06:40

Biology, 16.11.2020 06:40



Spanish, 16.11.2020 06:40

Mathematics, 16.11.2020 06:40

Computers and Technology, 16.11.2020 06:40


History, 16.11.2020 06:40


Mathematics, 16.11.2020 06:40

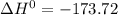 kJ/mol
kJ/mol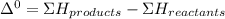
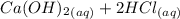 →
→ 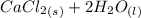
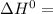 -1002.82 kJ/mol
-1002.82 kJ/mol![-17.2=[-795.8+2(285.85)]-[-1002.82+2\Delta H]](/tpl/images/1045/5683/27b56.png)