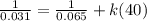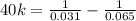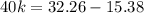Consider the following hypothetical aqueous reaction. A flask is charged with .065 mol of A in a total volume of 100.0 mL. The following data are collected:
Time (min) 0 10 20 30 40
Moles of A 0.065 0.051 0.042 0.036 0.031
This is a second order reaction.
What is the value of the rate constant for the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Consider the following hypothetical aqueous reaction. A flask is charged with .065 mol of A in a tot...
Questions


Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

History, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

History, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

English, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20



Mathematics, 30.04.2021 05:20

![r=k[A]^{x}[B]^{y}](/tpl/images/1045/5602/04c68.png)
![-\frac{d[A]}{dt} =k[A]^{2}](/tpl/images/1045/5602/5bd84.png)
![\frac{1}{[A]} =\frac{1}{[A]_{0}} +kt](/tpl/images/1045/5602/5ac7f.png)