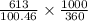Chemistry, 19.01.2021 19:30 holycow4916
A chemist dissolves 613 mg of pure perchloric acid in enough water to make up 360 mL of solution. Calculate the pH of the solution. Round your answer to 3 significant decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 09:30
Need , hurry pls create a superhero out of the element iron, what are its powers and his sidekick ( an element that works well with iron). how was the superhero made and who discovered him
Answers: 3

Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1
You know the right answer?
A chemist dissolves 613 mg of pure perchloric acid in enough water to make up 360 mL of solution. Ca...
Questions

History, 13.10.2020 02:01

Social Studies, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01

Arts, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


Social Studies, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


English, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01


English, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01

 .................1
.................1