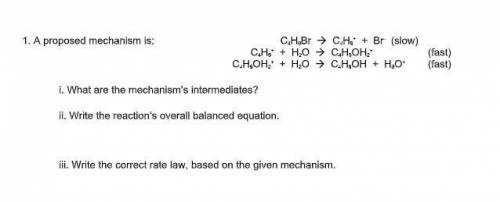
A proposed mechanism is:
C4H9Br --> C4H9^+ + Br^– (slow)
C 4H9^+ + H2O --> C4H9OH2^+ (f...

A proposed mechanism is:
C4H9Br --> C4H9^+ + Br^– (slow)
C 4H9^+ + H2O --> C4H9OH2^+ (fast)
C4H9OH2^+ + H2O --> C4H9OH + H3O^+ (fast)
i. What are the mechanism’s intermediates?
ii. Write the reaction’s overall balanced equation.
iii. Write the correct rate law, based on the given mechanism.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
Questions


Biology, 16.04.2020 21:40








Mathematics, 16.04.2020 21:40

English, 16.04.2020 21:40


Mathematics, 16.04.2020 21:40


Arts, 16.04.2020 21:40







