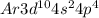Chemistry, 19.01.2021 14:00 zekiyekaya0571
Lewis dot structure for selenium and its oxidation number

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 14:50
Use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 +473 kj +206 kj -392 kj -91 kj -486 kj
Answers: 1

Chemistry, 24.06.2019 00:30
The ksp of srso4 is 3.2 × 10–7. what is the equilibrium concentration of sulfate ion in a 1.0-l solution of strontium sulfate to which 0.10 mol of sr(ch3co2)2 has been added? a. 3.2 × 10–8m b. 3.2 × 10–6m c. 5.7 × 10–4m d. 8.0 × 10–4m
Answers: 1

Chemistry, 24.06.2019 04:00
When studying the halogens (group 17) on the periodic table, how does the number of the valence electrons change as the energy level is increased? a) the total number of valence electrons does not change, it remains 7. b) the total number of valence electrons does not change, it remains 1. c) the total number of valence electrons increases by 1 with each energy level. d) the total number of valence electrons decreases by 1 with each energy level.
Answers: 1
You know the right answer?
Lewis dot structure for selenium and its oxidation number...
Questions













History, 03.10.2019 03:30



English, 03.10.2019 03:30

English, 03.10.2019 03:30

Mathematics, 03.10.2019 03:30