
In an experiment, potassium chlorate decomposed according to the following chemical equation.
KClO3 → KCl + O2
(Molar mass of KClO3 = 122.5 g/mol; KCl = 74.55 g/mol; O2 = 31.998 g/mol)
If the mass of potassium chlorate was 240 grams, which of the following calculations can be used to determine the mass of oxygen gas formed?
(240 × 2 × 31.998) ÷ (122.5 × 3) grams
(240 × 3 × 31.998) ÷ (122.5 × 2) grams
(240 × 2 × 122.5) ÷ (31.998 × 3) grams
(240 × 3 × 122.5) ÷ (31.998 × 2) grams

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

You know the right answer?
In an experiment, potassium chlorate decomposed according to the following chemical equation.
KClO3...
Questions

Advanced Placement (AP), 24.08.2021 01:00

Chemistry, 24.08.2021 01:00

Mathematics, 24.08.2021 01:00

Mathematics, 24.08.2021 01:00

Mathematics, 24.08.2021 01:00

Engineering, 24.08.2021 01:00





Chemistry, 24.08.2021 01:00


Mathematics, 24.08.2021 01:00


Mathematics, 24.08.2021 01:00

Mathematics, 24.08.2021 01:00


Mathematics, 24.08.2021 01:00


English, 24.08.2021 01:00

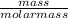
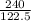
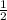 x 31.998
x 31.998

