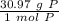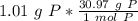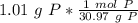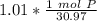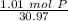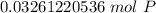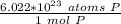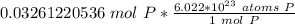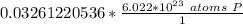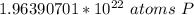Chemistry, 19.01.2021 06:00 TH3L0N3W0LF
How many atoms are present in a 1.01g sample of Phosphorus (P)? [Note: 1 mol = 6.022x10²³ atoms, molar mass of Phosphorus =30.97 g/mol) *
1 point
Captionless Image
3.48 x 10²¹ atoms
1.97 x 10²² atoms
1.04 x 10²⁶ atoms
3.36 x 10²⁴ atoms

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3


Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
How many atoms are present in a 1.01g sample of Phosphorus (P)? [Note: 1 mol = 6.022x10²³ atoms, mol...
Questions

English, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Chemistry, 14.09.2020 01:01

Biology, 14.09.2020 01:01

Chemistry, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Social Studies, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Chemistry, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Geography, 14.09.2020 01:01

English, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01