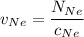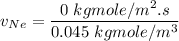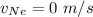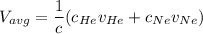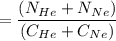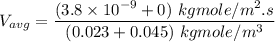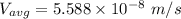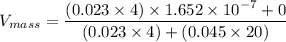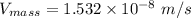Chemistry, 19.01.2021 05:30 Jadamachado45
In a gas-phase diffusion mass-transfer process, the steady-state flux of helium in a binary mixture of helium and neon is 3.8 x 10-9 kgmole/cm2 s, and the flux of neon is 0. At a particular point in the diffusion space, the concentration of helium is 0.023 kgmole/m3 and the concentration of neon is 0.045 kgmole/m3 . Estimate the individual net velocities of helium and neon along the direction of mass transfer, the average molar velocity, and the average mass velocity.''

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

You know the right answer?
In a gas-phase diffusion mass-transfer process, the steady-state flux of helium in a binary mixture...
Questions


English, 05.10.2019 18:00


Advanced Placement (AP), 05.10.2019 18:00

Physics, 05.10.2019 18:00

Chemistry, 05.10.2019 18:00

History, 05.10.2019 18:00


English, 05.10.2019 18:00

Mathematics, 05.10.2019 18:00

Mathematics, 05.10.2019 18:00




Mathematics, 05.10.2019 18:00



Mathematics, 05.10.2019 18:00

Physics, 05.10.2019 18:00

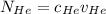
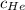 = concentration of Helium
= concentration of Helium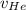 = net velocity of Helium
= net velocity of Helium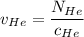
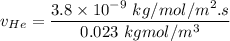
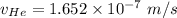
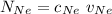
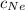 = Concentration of neon
= Concentration of neon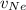 = net velocity of neon species
= net velocity of neon species