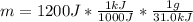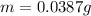Chemistry, 18.01.2021 22:40 Happypuppy1918
A certain chemical reaction releases 31.0 kj/g of heat for each gram of reactant consumed. How can you calculate what mass of reactant will produce of heat

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

You know the right answer?
A certain chemical reaction releases 31.0 kj/g of heat for each gram of reactant consumed. How can y...
Questions

Mathematics, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01

Social Studies, 16.10.2020 02:01


Mathematics, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01


Mathematics, 16.10.2020 02:01





Mathematics, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01



Mathematics, 16.10.2020 02:01


English, 16.10.2020 02:01