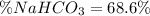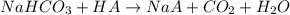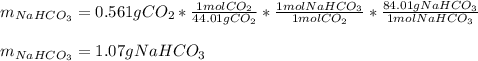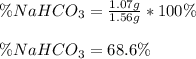Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
A mixture contains N a H C O 3 together with unreactive components. A 1.56 g sample of the mixture r...
Questions


Mathematics, 16.02.2021 09:00





Mathematics, 16.02.2021 09:00

Mathematics, 16.02.2021 09:00

Chemistry, 16.02.2021 09:00


Mathematics, 16.02.2021 09:00

Mathematics, 16.02.2021 09:00

Mathematics, 16.02.2021 09:10

Mathematics, 16.02.2021 09:10

Chemistry, 16.02.2021 09:10

Physics, 16.02.2021 09:10

Mathematics, 16.02.2021 09:10


Social Studies, 16.02.2021 09:10

Mathematics, 16.02.2021 09:10