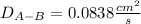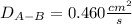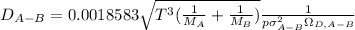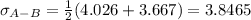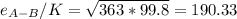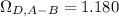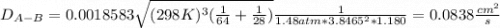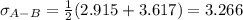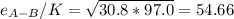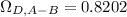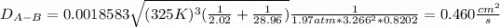Chemistry, 18.01.2021 21:10 KittyLoverCat
Estimate the value of the gas-phase diffusion coefficient for the following pairs using the Hirshfelder equation: a. Sulfur dioxide and nitrogen (N2) at 298 K and 1.5 x 105 Pa b. Hydrogen (H2) and air at 325 K and 2.0 x 105 Pa

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1


Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2
You know the right answer?
Estimate the value of the gas-phase diffusion coefficient for the following pairs using the Hirshfel...
Questions

Mathematics, 02.10.2019 15:50



Biology, 02.10.2019 15:50




History, 02.10.2019 15:50

Mathematics, 02.10.2019 15:50



History, 02.10.2019 15:50




Mathematics, 02.10.2019 15:50

History, 02.10.2019 15:50