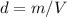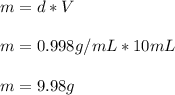Chemistry, 18.01.2021 21:10 gabriella1232002
The density of water may be taken to be 0.9980 g/mL at 21oC. Considering the volume of water that you used to prepare the the sodium chloride solution, calculate the mass in grams of water. Enter the mass to the nearest thousandth of a gram.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

You know the right answer?
The density of water may be taken to be 0.9980 g/mL at 21oC. Considering the volume of water that yo...
Questions

Computers and Technology, 29.06.2019 18:30


Spanish, 29.06.2019 18:30



History, 29.06.2019 18:30


History, 29.06.2019 18:30


English, 29.06.2019 18:30


History, 29.06.2019 18:30


Mathematics, 29.06.2019 18:30

Mathematics, 29.06.2019 18:30

Mathematics, 29.06.2019 18:30

History, 29.06.2019 18:30

Biology, 29.06.2019 18:30