

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
Iodine monochloride (ICl) has a higher boiling point than bromine (Br2) partly because iodine monoch...
Questions




Mathematics, 19.08.2020 23:01



Biology, 19.08.2020 23:01

Mathematics, 19.08.2020 23:01

Spanish, 19.08.2020 23:01

Chemistry, 19.08.2020 23:01


Mathematics, 19.08.2020 23:01

Mathematics, 19.08.2020 23:01

English, 19.08.2020 23:01

Mathematics, 19.08.2020 23:01

Computers and Technology, 19.08.2020 23:01


Computers and Technology, 19.08.2020 23:01

English, 19.08.2020 23:01

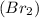 is a non polar molecule as there is no electronegativity difference between two bromine atoms. The molecules are bonded by weak vanderwaal forces and thus has low boiling point.
is a non polar molecule as there is no electronegativity difference between two bromine atoms. The molecules are bonded by weak vanderwaal forces and thus has low boiling point.



