
Chemistry, 18.01.2021 16:50 dragongacha777
PLEASE HELP ME
1. What is the molality of a solution made up of 43.6 mol of CaCl2 dissolved by 13.5 kg of water?
2. How many moles of Na2CO3 are required to create 9.54 liters of a 3.4 M solution?
3. Which of these actions can result in decreasing the molarity of a solution?
Select all that apply.
A
adding solute
B
adding solvent
C
removing solute
D
removing solvent
Thanks Y'all:)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
PLEASE HELP ME
1. What is the molality of a solution made up of 43.6 mol of CaCl2 dissolved by 13.5...
Questions

Mathematics, 12.05.2021 14:00

Mathematics, 12.05.2021 14:00





History, 12.05.2021 14:00

Mathematics, 12.05.2021 14:00



Mathematics, 12.05.2021 14:00

History, 12.05.2021 14:00

History, 12.05.2021 14:00


Chemistry, 12.05.2021 14:00

Mathematics, 12.05.2021 14:00

History, 12.05.2021 14:00

Mathematics, 12.05.2021 14:00


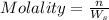
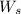 = weight of solvent
= weight of solvent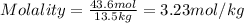
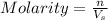
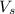 = volume of solution in L
= volume of solution in L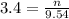
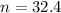
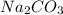 is 32.4
is 32.4 

