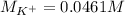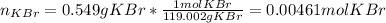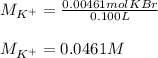Chemistry, 18.01.2021 14:00 makayyafreeman
Suppose of potassium bromide is dissolved in of a aqueous solution of silver nitrate. Calculate the final molarity of potassium cation in the solution. You can assume the volume of the solution doesn't change when the potassium bromide is dissolved in it. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

You know the right answer?
Suppose of potassium bromide is dissolved in of a aqueous solution of silver nitrate. Calculate the...
Questions



Computers and Technology, 18.11.2020 23:20

Mathematics, 18.11.2020 23:20








Arts, 18.11.2020 23:20

Chemistry, 18.11.2020 23:20



History, 18.11.2020 23:20

Mathematics, 18.11.2020 23:20