
Chemistry, 17.01.2021 18:40 paulitaaustin
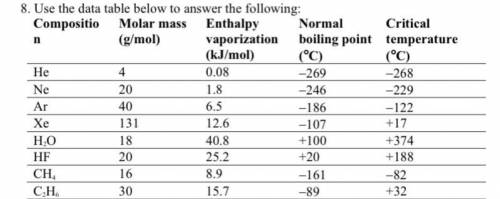
A. Among nonpolar liquids, those with higher molar masses tend to have normal boiling points that are (higher, lower, or about the same).
b. Among compounds of approximately the same molar mass, those with greater polarities tend to have enthalpies of vaporization that are (higher, lower, or about the same).
c. Which is the only noble gas listed that is stable as a liquid at 0°C? Explain your answer using the concept of critical temperature.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
A. Among nonpolar liquids, those with higher molar masses tend to have normal boiling points that ar...
Questions


Social Studies, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40

Social Studies, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40


Mathematics, 10.05.2021 17:40


Mathematics, 10.05.2021 17:40

English, 10.05.2021 17:40

Health, 10.05.2021 17:40


Biology, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40

English, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40

Social Studies, 10.05.2021 17:40




