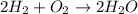Chemistry, 16.01.2021 16:20 amandafutch24
1. How is the atom count for each element on the reactant side of a balanced chemical equation related to the atom count for each element on the product side of the same equation?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1
You know the right answer?
1. How is the atom count for each element on the reactant side of a balanced chemical equation relat...
Questions


Mathematics, 18.03.2021 02:40

English, 18.03.2021 02:40

Health, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


English, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40


English, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Biology, 18.03.2021 02:40