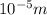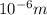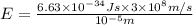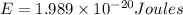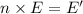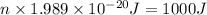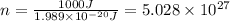Chemistry, 21.01.2020 11:31 bapehoodboi
Calculate thr number of photons having a wavelength of 10.0 μm required to produce 1.0 kj of energy.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Calculate thr number of photons having a wavelength of 10.0 μm required to produce 1.0 kj of energy....
Questions




Chemistry, 11.11.2020 04:40


Mathematics, 11.11.2020 04:40

Health, 11.11.2020 04:40


History, 11.11.2020 04:40

Mathematics, 11.11.2020 04:40

English, 11.11.2020 04:40




Mathematics, 11.11.2020 04:40

Biology, 11.11.2020 04:40

Business, 11.11.2020 04:40


Chemistry, 11.11.2020 04:40

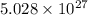 .
.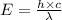
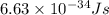
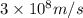
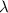 = wavelength = 10.0 μm =
= wavelength = 10.0 μm =