Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
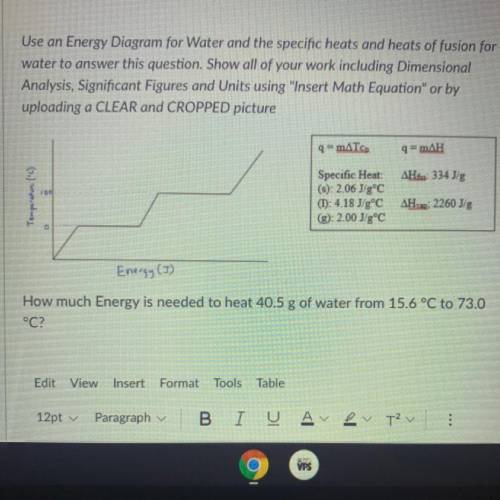
Thermodynamics and Q
How much energy is needed to heat 40.5g of water from 15.6°C to 73.0°C <...
How much energy is needed to heat 40.5g of water from 15.6°C to 73.0°C <...
Questions

Mathematics, 27.01.2020 22:31

Chemistry, 27.01.2020 22:31

Social Studies, 27.01.2020 22:31











Mathematics, 27.01.2020 22:31



Mathematics, 27.01.2020 22:31

Spanish, 27.01.2020 22:31

Business, 27.01.2020 22:31