Consider the following reaction:
10 KCIO3 + 3 P4 → 3 P4010 + 10 KCI
Given 30 moles of P4 and...

Chemistry, 15.01.2021 18:20 rosemary909
Consider the following reaction:
10 KCIO3 + 3 P4 → 3 P4010 + 10 KCI
Given 30 moles of P4 and 86 moles of KCIO3, which would act as the limiting reactant?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
Questions






History, 14.07.2019 11:00

English, 14.07.2019 11:00


History, 14.07.2019 11:00


Mathematics, 14.07.2019 11:00

Mathematics, 14.07.2019 11:00


Mathematics, 14.07.2019 11:00


Chemistry, 14.07.2019 11:00

History, 14.07.2019 11:00



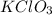 is the limiting reactant in the given reaction.
is the limiting reactant in the given reaction.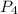 = 30 moles
= 30 moles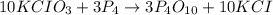
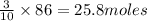 of
of 

