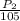Chemistry, 15.01.2021 16:20 Savageboyn
A gas at 76 K is under a pressure of 78.4 kPa. If the temperature changes to 105 what will be the new pressure?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
A gas at 76 K is under a pressure of 78.4 kPa. If the temperature changes to 105 what will be the ne...
Questions

Physics, 01.04.2020 19:38

Mathematics, 01.04.2020 19:38

History, 01.04.2020 19:38


Mathematics, 01.04.2020 19:38


Physics, 01.04.2020 19:38


Mathematics, 01.04.2020 19:38



History, 01.04.2020 19:38

Mathematics, 01.04.2020 19:38


English, 01.04.2020 19:38



History, 01.04.2020 19:38


Mathematics, 01.04.2020 19:38

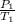 =
= 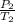
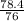 =
=